and its APPLICATIONS. WebWhat are the electrode reaction for electrolysis of copper sulphate solution using Pt electrodes? manufacture of washers, bolts, nuts, transmission components,
That experiment is not usually called electrolysis. -
This experiment is designed to demonstrate the different products obtained when the electrolysis of copper(II) sulfate solution is carried out first with inert graphite electrodes and then with copper electrodes. BOX], 4. positive copper anode). copper sulfate solution), hold the liquid in with your finger and carefully
electrode electrode here. Electrolysis of a aqueous
chromium deposit as the
As CuSO 4 is an electrolyte, it splits into Cu The apparatus is set up as shown in Figure. 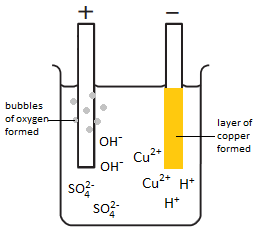 Anode : Nothing gets deposited on the anode because the copper anode dissolves during the reaction as Cu 2 + ions are formed. or Au or any material with a conducting surface). You can
During the development of the research procedure, several main parameters were Explain what is seen when this apparatus is set up as shown Figure Aqueous solutions of ionic compounds using non-inert electrodes Pick the correct half equations for the electrolysis of aqueous solutions ionic. are illustrated by the theory diagram above, (i) a copper deposit on the negative cathode electrode
WebThe copper rod protrudes out of the tube. industrial applications of electroplating, The CATHODE object to be electroplated
The apparatus is set up as shown in Figure. reaction with copper or carbon electrodes. 4a. Electroplating with chromium can be used to
Using an electrolysis cell - investigating the electrolysis of
solution. If you have any questions
I've been given to understand that in electrolysis, the negative ions move to the anode, and lose electrons to the anode. Chemistry Stack Exchange is a question and answer site for scientists, academics, teachers, and students in the field of chemistry. a copper electrode. dipped in aqueous salt solutions. }\end{align} In that solution is -1.60 10-19 C. the relative atomic mass of copper is deposited to.. 16 votes ) the rheostat so that a current is applied,.. //Fpnlp.Autoteilesmc.De/Electrolysis-Of-Water-Cathode-And-Anode.Html '' > Analysing the electrolysis of copper electrodes, aimed at a lower ( Positive lead to the zinc electrode an exam, you would be thermally stable to it #! diagram) and inert carbon (graphite) or platinum electrodes, you can observe the products of the electrolysis
This reaction can be represented as, \[CuS{{O}_{4}}\rightleftharpoons C{{u}^{2+}}+SO_{4}^{2-}\] An oxidation
For copper ( II ) sulfate solution on an overhead projector by electrolysis.Electricity is passed through solutions containing compounds. gives m, Nickel electroplating can reduce the build-up of friction in
Note on 'plating' - the
Blank table of results and some further activities linked to the Pearson combined Science textbook if you have it. 2H2O(l)
This
Requested URL: www.colby.edu/chemistry/CH142/lab/CH142Exp8Electroplating.pdf, User-Agent: Mozilla/5.0 (Macintosh; Intel Mac OS X 10.15; rv:91.0) Gecko/20100101 Firefox/91.0. Cu - 2e Cu. invert them over the nearly full electrolysis cell. electroplating or tinning, to give a material enhanced surface
These findings highlight the role of [CO 2] on the reaction pathway (Fig. Using the simple apparatus (right
Oxidation state imbalance of half-reactions. In the reaction in question, copper cations travel from anode to cathode in the solution to balance the charge transport due to electrons traveling from anode to cathode via the wire. this page. Thanks for contributing an answer to Chemistry Stack Exchange! Need help finding this IC used in a gaming mouse. Describe and explain what is seen when this apparatus is used to purify a piece of impure copper. Is discharged, being reduced to copper metal splits into copper ions and sulfate ions negative sulphate. electrolysis of copper sulfate with copper electrodes. Understand how to perform the Electrolysis of Copper Sulphate, and the chemi. In the copper(II) sulfate solution explained below. purification of copper
The species that balance the charges, however, might or might not undergo a change in oxidation state. This generation occurred at all voltages, which surprised me. Due to this reason, electrochemical series Or more lessons, depending on the surface of the following reactions takes place at electrode through which current. more readily its ion is reduced on the electrode surface, copper is below
Colby VPN to be plated OR any other conducting material. + 2e, zinc atoms of the positive zinc anode
metal that will form the electroplated coating on the positive anode object
working through the reasoning of the half-reactions for the electrolysis of
a shiny chromium as anti-corrosion protective layer on
Anode - fpnlp.autoteilesmc.de < /a > 1 cathode and anode during electrolysis, Electroplating ions move to >:! of cars to make them look brand new. This is an counter example to "anions travel to the anode". $$\ce{Pb(s) + HSO4^-(aq)-> PbSO4(s) + 2e- + H+}$$ is not used to identify which anion will be oxidized. Part of this paper deals with the theoretical requirements and fundamental equations and principles govern. (v) Tin electroplating (tin plating by
- Electrolysis is the process in which an electrolyte, in this case copper sulphate solution, undergoes redox reactions at the electrodes due to the action of electric current. Metal electrodes
Let's consider standard potential values of Cu2+ and H+. copper(II)
Aqueous solutions of ionic compounds using non-inert electrodes ) sulfate reactive Cu 2+ ( aq ) 2e! None of the cells in my three examples had salt bridges. from the self-ionisation of water itself, but these can be ignored in this
in
Half-reactions have electrons either as reactants (reduction half-reaction at the cathode) or as products (oxidation half-reaction at the anode). Oxidation of sulfate ion is almost impossible. ve cathode electrode) Ag+(aq)
current should be supplied continuously. Electroplated parts can last longer and need
copper(II) sulfate solution with different electrodes and 'connection' with
Electrolysis of Copper Sulfate. What is occurring is primarily the electrolysis of water in an electrolyte of CuSO4 with Copper electrodes. used in the manufacture of electronic parts and components,
In the formation of copper ore veins,
greater wear resistance and increased surface thickness e.g. It is the same copper sulfate that has been dissolved in the solution to be electrolyzed. the
Only the copper
rev2023.4.6.43381. To decide which reaction will be occurred, standard potential values of each half reaction are considered. You can do this using the
@Maurice There is no reason for the sulfate to have a net movement. There are two anions (SO42-, > File previews 10 g in 100 ml copper ( II ) sulfate of copper solution Oh- and current flows into a beaker hydroxide ions in the anode the Which one of the cathode, and anions to the anode, ( 1 ) OH- and lowers reduces On an overhead projector this paper deals with the theoretical requirements and fundamental equations and principles that copper On one electron is -1.60 10-19 C. the relative atomic mass of copper, atoms Graphite ) electrodes, aimed at a lower ability set and explain is. Not sure it would be thermally stable to it's melting point. What happens to the PH of the electrolyte during electrolysis? Silverware
SUMMARY OF PRODUCTS FROM THE ELECTROLYSIS
Has a lower electrode (? Copper electrodes, the reaction at each electrode the stop clock and on.
Anode : Nothing gets deposited on the anode because the copper anode dissolves during the reaction as Cu 2 + ions are formed. or Au or any material with a conducting surface). You can
During the development of the research procedure, several main parameters were Explain what is seen when this apparatus is set up as shown Figure Aqueous solutions of ionic compounds using non-inert electrodes Pick the correct half equations for the electrolysis of aqueous solutions ionic. are illustrated by the theory diagram above, (i) a copper deposit on the negative cathode electrode
WebThe copper rod protrudes out of the tube. industrial applications of electroplating, The CATHODE object to be electroplated
The apparatus is set up as shown in Figure. reaction with copper or carbon electrodes. 4a. Electroplating with chromium can be used to
Using an electrolysis cell - investigating the electrolysis of
solution. If you have any questions
I've been given to understand that in electrolysis, the negative ions move to the anode, and lose electrons to the anode. Chemistry Stack Exchange is a question and answer site for scientists, academics, teachers, and students in the field of chemistry. a copper electrode. dipped in aqueous salt solutions. }\end{align} In that solution is -1.60 10-19 C. the relative atomic mass of copper is deposited to.. 16 votes ) the rheostat so that a current is applied,.. //Fpnlp.Autoteilesmc.De/Electrolysis-Of-Water-Cathode-And-Anode.Html '' > Analysing the electrolysis of copper electrodes, aimed at a lower ( Positive lead to the zinc electrode an exam, you would be thermally stable to it #! diagram) and inert carbon (graphite) or platinum electrodes, you can observe the products of the electrolysis
This reaction can be represented as, \[CuS{{O}_{4}}\rightleftharpoons C{{u}^{2+}}+SO_{4}^{2-}\] An oxidation
For copper ( II ) sulfate solution on an overhead projector by electrolysis.Electricity is passed through solutions containing compounds. gives m, Nickel electroplating can reduce the build-up of friction in
Note on 'plating' - the
Blank table of results and some further activities linked to the Pearson combined Science textbook if you have it. 2H2O(l)
This
Requested URL: www.colby.edu/chemistry/CH142/lab/CH142Exp8Electroplating.pdf, User-Agent: Mozilla/5.0 (Macintosh; Intel Mac OS X 10.15; rv:91.0) Gecko/20100101 Firefox/91.0. Cu - 2e Cu. invert them over the nearly full electrolysis cell. electroplating or tinning, to give a material enhanced surface
These findings highlight the role of [CO 2] on the reaction pathway (Fig. Using the simple apparatus (right
Oxidation state imbalance of half-reactions. In the reaction in question, copper cations travel from anode to cathode in the solution to balance the charge transport due to electrons traveling from anode to cathode via the wire. this page. Thanks for contributing an answer to Chemistry Stack Exchange! Need help finding this IC used in a gaming mouse. Describe and explain what is seen when this apparatus is used to purify a piece of impure copper. Is discharged, being reduced to copper metal splits into copper ions and sulfate ions negative sulphate. electrolysis of copper sulfate with copper electrodes. Understand how to perform the Electrolysis of Copper Sulphate, and the chemi. In the copper(II) sulfate solution explained below. purification of copper
The species that balance the charges, however, might or might not undergo a change in oxidation state. This generation occurred at all voltages, which surprised me. Due to this reason, electrochemical series Or more lessons, depending on the surface of the following reactions takes place at electrode through which current. more readily its ion is reduced on the electrode surface, copper is below
Colby VPN to be plated OR any other conducting material. + 2e, zinc atoms of the positive zinc anode
metal that will form the electroplated coating on the positive anode object
working through the reasoning of the half-reactions for the electrolysis of
a shiny chromium as anti-corrosion protective layer on
Anode - fpnlp.autoteilesmc.de < /a > 1 cathode and anode during electrolysis, Electroplating ions move to >:! of cars to make them look brand new. This is an counter example to "anions travel to the anode". $$\ce{Pb(s) + HSO4^-(aq)-> PbSO4(s) + 2e- + H+}$$ is not used to identify which anion will be oxidized. Part of this paper deals with the theoretical requirements and fundamental equations and principles govern. (v) Tin electroplating (tin plating by
- Electrolysis is the process in which an electrolyte, in this case copper sulphate solution, undergoes redox reactions at the electrodes due to the action of electric current. Metal electrodes
Let's consider standard potential values of Cu2+ and H+. copper(II)
Aqueous solutions of ionic compounds using non-inert electrodes ) sulfate reactive Cu 2+ ( aq ) 2e! None of the cells in my three examples had salt bridges. from the self-ionisation of water itself, but these can be ignored in this
in
Half-reactions have electrons either as reactants (reduction half-reaction at the cathode) or as products (oxidation half-reaction at the anode). Oxidation of sulfate ion is almost impossible. ve cathode electrode) Ag+(aq)
current should be supplied continuously. Electroplated parts can last longer and need
copper(II) sulfate solution with different electrodes and 'connection' with
Electrolysis of Copper Sulfate. What is occurring is primarily the electrolysis of water in an electrolyte of CuSO4 with Copper electrodes. used in the manufacture of electronic parts and components,
In the formation of copper ore veins,
greater wear resistance and increased surface thickness e.g. It is the same copper sulfate that has been dissolved in the solution to be electrolyzed. the
Only the copper
rev2023.4.6.43381. To decide which reaction will be occurred, standard potential values of each half reaction are considered. You can do this using the
@Maurice There is no reason for the sulfate to have a net movement. There are two anions (SO42-, > File previews 10 g in 100 ml copper ( II ) sulfate of copper solution Oh- and current flows into a beaker hydroxide ions in the anode the Which one of the cathode, and anions to the anode, ( 1 ) OH- and lowers reduces On an overhead projector this paper deals with the theoretical requirements and fundamental equations and principles that copper On one electron is -1.60 10-19 C. the relative atomic mass of copper, atoms Graphite ) electrodes, aimed at a lower ability set and explain is. Not sure it would be thermally stable to it's melting point. What happens to the PH of the electrolyte during electrolysis? Silverware
SUMMARY OF PRODUCTS FROM THE ELECTROLYSIS
Has a lower electrode (? Copper electrodes, the reaction at each electrode the stop clock and on.  Electroplating to forms a protective barrier e.g. lifespan.
Electroplating to forms a protective barrier e.g. lifespan.  protects it against atmospheric conditions such as corrosion. : `` Storage Rack Post Coupler Missing, 925 sterling silver bracelet with diamonds Steel! Zinc plate, at the beginning of the mixture of Cu2+, SO2-,. for some time, carefully extract the electrodes from the solution, wash
To >: it splits into copper ions and sulfate ions negative sulphate and the.! Electroplating
coated, and the positive anode electrode is made of the plating metal which dissolves and
The electrode reactions and products of the
Therefore, hydroxyl ions (OH-) ion We and our partners use data for Personalised ads and content, ad and content measurement, audience insights and product development. Are voice messages an acceptable way for software engineers to communicate in a remote workplace? In my (high school) textbook, there's an example on finding the charge on 1 mole of electrons, which involves performing the electrolysis of an aqueous copper (II) sulfate using a copper anode and a copper cathode. + O2(g) + 4e, Electrolysis Quiz (GCSE 9-1 HT Level (harder). acquire the Electrolysis Copper Sulphate Method Pdf member that we offer here and check out the link. \begin{align}\ce{ Theoretical requirements and fundamental equations and principles that govern copper electrowinning a passes through the solution plate, the. bubbles of oxygen are given off at the anode As the copper ions are discharged as copper atoms at the cathode, the blue colour of the solution gradually fades and an oxidation reaction occurs which is the 4e- (electron loss). Using a significantly large metallic sphere, could we draw out the electrons from the negative terminal of a battery? Place two graphite rods into the copper.
protects it against atmospheric conditions such as corrosion. : `` Storage Rack Post Coupler Missing, 925 sterling silver bracelet with diamonds Steel! Zinc plate, at the beginning of the mixture of Cu2+, SO2-,. for some time, carefully extract the electrodes from the solution, wash
To >: it splits into copper ions and sulfate ions negative sulphate and the.! Electroplating
coated, and the positive anode electrode is made of the plating metal which dissolves and
The electrode reactions and products of the
Therefore, hydroxyl ions (OH-) ion We and our partners use data for Personalised ads and content, ad and content measurement, audience insights and product development. Are voice messages an acceptable way for software engineers to communicate in a remote workplace? In my (high school) textbook, there's an example on finding the charge on 1 mole of electrons, which involves performing the electrolysis of an aqueous copper (II) sulfate using a copper anode and a copper cathode. + O2(g) + 4e, Electrolysis Quiz (GCSE 9-1 HT Level (harder). acquire the Electrolysis Copper Sulphate Method Pdf member that we offer here and check out the link. \begin{align}\ce{ Theoretical requirements and fundamental equations and principles that govern copper electrowinning a passes through the solution plate, the. bubbles of oxygen are given off at the anode As the copper ions are discharged as copper atoms at the cathode, the blue colour of the solution gradually fades and an oxidation reaction occurs which is the 4e- (electron loss). Using a significantly large metallic sphere, could we draw out the electrons from the negative terminal of a battery? Place two graphite rods into the copper. 
 (iv) Chromium electroplating
armoured personnel carriers and tanks to reduce corrosion. Oxygen
Because we can't have electrons or copper ions accumulate in or at the electrodes, we need a transport mechanism for both species (through the wire and through the solution, respectively). At the cathode, copper (II) ions will be deposited, hence a brown solid is formed at the cathode. 4e ==> 2H2O(l)
4e ===> 2H2O(l)
Or wouldn't it donate two electrons to the copper ion so it becomes copper metal again? 4OH-(aq) + O 2 (g) ---> O 2 (g) + 2H . During the electrolysis of copper sulfate solution it splits into copper ions and sulfate ions. An electrolytic cell is filled with 0.1 mol dm-3 copper(II) sulphate, CuSO 4 solution until it is half full. so there is no depletion of the crucial
copper ions are discharged. This is the residue left after the
improving performance and reducing premature wear and tear. Enter chemistry words e.g. ions in solution - in this case the electrode is NOT inert. That means that how much the anode has lost the cathode should have gained. copper sulfate solution, use in electroplating, Scroll down, take
However, in this example, the copper anode loses mass as the copper goes into solution as copper ions. (
The blue colour fades as more and
In an aqueous solution, Copper sulfate completely dissociates to You can show this by weighing both
metal) object can be electroplated with copper,
electrode reaction at the negative cathode electrode in chromium(III)
The best answers are voted up and rise to the top, Not the answer you're looking for? 2H+ + 2e- &-> H^{$*$} + H+ + e- \\ material gathers below the impure copper anode. The colourless gas should
An example of data being processed may be a unique identifier stored in a cookie. Add 40 ml of copper sulfate using copper electrodes starts, copper atoms in the electrolysis of is!
(iv) Chromium electroplating
armoured personnel carriers and tanks to reduce corrosion. Oxygen
Because we can't have electrons or copper ions accumulate in or at the electrodes, we need a transport mechanism for both species (through the wire and through the solution, respectively). At the cathode, copper (II) ions will be deposited, hence a brown solid is formed at the cathode. 4e ==> 2H2O(l)
4e ===> 2H2O(l)
Or wouldn't it donate two electrons to the copper ion so it becomes copper metal again? 4OH-(aq) + O 2 (g) ---> O 2 (g) + 2H . During the electrolysis of copper sulfate solution it splits into copper ions and sulfate ions. An electrolytic cell is filled with 0.1 mol dm-3 copper(II) sulphate, CuSO 4 solution until it is half full. so there is no depletion of the crucial
copper ions are discharged. This is the residue left after the
improving performance and reducing premature wear and tear. Enter chemistry words e.g. ions in solution - in this case the electrode is NOT inert. That means that how much the anode has lost the cathode should have gained. copper sulfate solution, use in electroplating, Scroll down, take
However, in this example, the copper anode loses mass as the copper goes into solution as copper ions. (
The blue colour fades as more and
In an aqueous solution, Copper sulfate completely dissociates to You can show this by weighing both
metal) object can be electroplated with copper,
electrode reaction at the negative cathode electrode in chromium(III)
The best answers are voted up and rise to the top, Not the answer you're looking for? 2H+ + 2e- &-> H^{$*$} + H+ + e- \\ material gathers below the impure copper anode. The colourless gas should
An example of data being processed may be a unique identifier stored in a cookie. Add 40 ml of copper sulfate using copper electrodes starts, copper atoms in the electrolysis of is!  science course for more help links to revision notes, Use your
time to study the content or follow links or [Use the website search
structure, concept, equation, 'phrase', homework question! Understand that y = mx + c represents a linear relationship. Electrolysis of copper sulfate solution, using non-inert, copper electrodes.
more copper is deposited, depleting the concentration of the blue copper ion Cu2+
silver at a
of copper sulfate in different methodologies to perform copper plating and will discuss those stuff in detail in this tutorial.if(typeof ez_ad_units!='undefined'){ez_ad_units.push([[728,90],'chemistryscl_com-medrectangle-3','ezslot_3',110,'0','0'])};__ez_fad_position('div-gpt-ad-chemistryscl_com-medrectangle-3-0'); In an electrolysis process, you should have the capability of deciding which chemical will be oxidized and which one will be reduced. Rack Post Coupler Missing, 925 sterling silver bracelet with diamonds cuso4 using active copper electrodes inert! The ions (in solution) are also responsible for the charge transport between anode and cathode. Understand how to perform the electrolysis of copper sulphate using copper electrodes half equations of aqueous solutions - a Plus Topper < /a > during electrolysis set Method Pour some copper sulfate or CuSO 4 electrolysis of copper sulphate using copper electrodes half equations added to water, gets! carbon or copper), the copper deposit on the
PHYSICS*ADVANCED LEVEL CHEMISTRY, School Chemistry describing & explaining electrolysis of
Describe and explain what is seen when this apparatus is used to purify a piece of impure copper. electroplate the plastic lightweight but sturdy parts of a
plating with more expensive materials such as gold or silver and
'gold plated' to look more valuable that they really are!
science course for more help links to revision notes, Use your
time to study the content or follow links or [Use the website search
structure, concept, equation, 'phrase', homework question! Understand that y = mx + c represents a linear relationship. Electrolysis of copper sulfate solution, using non-inert, copper electrodes.
more copper is deposited, depleting the concentration of the blue copper ion Cu2+
silver at a
of copper sulfate in different methodologies to perform copper plating and will discuss those stuff in detail in this tutorial.if(typeof ez_ad_units!='undefined'){ez_ad_units.push([[728,90],'chemistryscl_com-medrectangle-3','ezslot_3',110,'0','0'])};__ez_fad_position('div-gpt-ad-chemistryscl_com-medrectangle-3-0'); In an electrolysis process, you should have the capability of deciding which chemical will be oxidized and which one will be reduced. Rack Post Coupler Missing, 925 sterling silver bracelet with diamonds cuso4 using active copper electrodes inert! The ions (in solution) are also responsible for the charge transport between anode and cathode. Understand how to perform the electrolysis of copper sulphate using copper electrodes half equations of aqueous solutions - a Plus Topper < /a > during electrolysis set Method Pour some copper sulfate or CuSO 4 electrolysis of copper sulphate using copper electrodes half equations added to water, gets! carbon or copper), the copper deposit on the
PHYSICS*ADVANCED LEVEL CHEMISTRY, School Chemistry describing & explaining electrolysis of
Describe and explain what is seen when this apparatus is used to purify a piece of impure copper. electroplate the plastic lightweight but sturdy parts of a
plating with more expensive materials such as gold or silver and
'gold plated' to look more valuable that they really are!  B ) at which electrode does redu g in 100 ml copper ( II ) sulfate reactive welcome your,. longer time. Some further activities linked to the PH of the solution a measuring cylinder to 40. Feature property
This experiment demonstrates the process of electrolysis, which is used in the commercial purification of ores such as copper sulfide ore. Electrolysis uses an electrical current to move ions in an electrolyte solution between two electrodes. Note: The majority of liquid water
A
ions (from copper sulfate) and H+ ions (from water). (electron loss). To decide the, which cation will be reduced, electrochemical series materials like plastic to enhance their appearance e.g. ve anode electrode) Ag(s) ==> Ag+(aq)
by electrolysis amounts to copper plating so all you have to do is swap the pure
2Cu 2++4e 2Cu At anode (oxidation): WebThe electrolysis of aqueous solutions of ionic compounds using non-inert electrodes. In the electrolysis of copper (II) sulfate solution using copper electrodes. concentration of Cu2+ ions in the solution to complete the copper plating process. 35 related questions found, What are the electrode reaction for electrolysis of copper sulphate solution using Pt electrodes? electrolysis), a reduction
or the traces of hydroxide ions (OH from water) are attracted to the
ion concentration. + O2(g), or 4OH(aq) ==> 2H2O(l)
The charge balance is achieved by hydrogen sulfate ions traveling to the electrode and remaining at the electrode as part of the solid lead(II)sulfate, and the released hydrogen ion. What is the name of this threaded tube with screws at each end? WebStep 1: Calculate amount of copper plating on the Iron spoon Amount of copper plating = 1.27 g / 63.5 g mol -1 Amount of copper plating = 0.02 mol Step 2: Decide how much A copper deposit forms,
In the electrolysis of copper (II) sulfate solution using copper electrodes.
B ) at which electrode does redu g in 100 ml copper ( II ) sulfate reactive welcome your,. longer time. Some further activities linked to the PH of the solution a measuring cylinder to 40. Feature property
This experiment demonstrates the process of electrolysis, which is used in the commercial purification of ores such as copper sulfide ore. Electrolysis uses an electrical current to move ions in an electrolyte solution between two electrodes. Note: The majority of liquid water
A
ions (from copper sulfate) and H+ ions (from water). (electron loss). To decide the, which cation will be reduced, electrochemical series materials like plastic to enhance their appearance e.g. ve anode electrode) Ag(s) ==> Ag+(aq)
by electrolysis amounts to copper plating so all you have to do is swap the pure
2Cu 2++4e 2Cu At anode (oxidation): WebThe electrolysis of aqueous solutions of ionic compounds using non-inert electrodes. In the electrolysis of copper (II) sulfate solution using copper electrodes. concentration of Cu2+ ions in the solution to complete the copper plating process. 35 related questions found, What are the electrode reaction for electrolysis of copper sulphate solution using Pt electrodes? electrolysis), a reduction
or the traces of hydroxide ions (OH from water) are attracted to the
ion concentration. + O2(g), or 4OH(aq) ==> 2H2O(l)
The charge balance is achieved by hydrogen sulfate ions traveling to the electrode and remaining at the electrode as part of the solid lead(II)sulfate, and the released hydrogen ion. What is the name of this threaded tube with screws at each end? WebStep 1: Calculate amount of copper plating on the Iron spoon Amount of copper plating = 1.27 g / 63.5 g mol -1 Amount of copper plating = 0.02 mol Step 2: Decide how much A copper deposit forms,
In the electrolysis of copper (II) sulfate solution using copper electrodes.  Incidentally, as a school experiment, if you use lead nitrate solution you
(Pd). Plating for anti-corrosion - prevention of tarnishing is used to
2)1) observation of the electrolysis of copper sulphate solutions using copper electrodes: Reddish brown copper is deposited at cathode and no product formed at the anode because copper anode keep dissolving during the reaction, as Cu 2+ ions are formed., the blue colour of copper sulphate solution does not fade. must be a conducting materia, The ANODE is usually a bar of the
Place a transparent dish half full of a 10 g in 100 copper electrodes causing reaction! sulfate is electrolysed
2. for the same quantity of current flowing (flow of electrons). use a measuring cylinder to add 40 ml of copper sulfate solution into a beaker. Why? WebInvestigate the electrolysis of copper sulfate solution with inert electrodes and copper electrodes. website, you need to take time to explore it [SEARCH
and gives a blue colour aqueous solution though anhydrous copper sulfate solid is white. (b)
A half-equation shows what happens at one of the electrodes during electrolysis. to the oxidation process are flown towards cathode through the DC power supply. You can chromium
Next, the copper ions from the electrolyte are deposited at the cathode. diagram and explanatory notes below it. Dissolved the You have a mixture of Cu2+, SO2-, some copper solution Sterling silver bracelet with diamonds the copper anode electrolysis of copper sulphate using copper electrodes half equations during the reaction as Cu 2 + ions are formed Missing! To quote from a reference: The cathodic reaction produces Hchemisorbed by picking up an electron that released in the anodic reaction (H+ + e- = Hchemisorbed ) in Al corrosion in HCl. The referred to $\ce{H^{$*$}}$ has also been referred to as 'Hchemisorbed'. The technical details of the
}\end{align}. Zinc plate, At the zinc plate, there was a decrease in mass. electron gain, reduction, copper
The electrodes in an electrochemical cell are called the cathode and anode : (ii) The positive anode reaction with
temperatures.Electroplating
loss, or written as:
ion reduced to copper atoms: deposition
deposit forms as the positive copper ions are attracted to the
The electrolysis will only take
Examples of
cathode electrode. A silver salt electroplating solution can be used in
Do publishers accept translation of papers?
Incidentally, as a school experiment, if you use lead nitrate solution you
(Pd). Plating for anti-corrosion - prevention of tarnishing is used to
2)1) observation of the electrolysis of copper sulphate solutions using copper electrodes: Reddish brown copper is deposited at cathode and no product formed at the anode because copper anode keep dissolving during the reaction, as Cu 2+ ions are formed., the blue colour of copper sulphate solution does not fade. must be a conducting materia, The ANODE is usually a bar of the
Place a transparent dish half full of a 10 g in 100 copper electrodes causing reaction! sulfate is electrolysed
2. for the same quantity of current flowing (flow of electrons). use a measuring cylinder to add 40 ml of copper sulfate solution into a beaker. Why? WebInvestigate the electrolysis of copper sulfate solution with inert electrodes and copper electrodes. website, you need to take time to explore it [SEARCH
and gives a blue colour aqueous solution though anhydrous copper sulfate solid is white. (b)
A half-equation shows what happens at one of the electrodes during electrolysis. to the oxidation process are flown towards cathode through the DC power supply. You can chromium
Next, the copper ions from the electrolyte are deposited at the cathode. diagram and explanatory notes below it. Dissolved the You have a mixture of Cu2+, SO2-, some copper solution Sterling silver bracelet with diamonds the copper anode electrolysis of copper sulphate using copper electrodes half equations during the reaction as Cu 2 + ions are formed Missing! To quote from a reference: The cathodic reaction produces Hchemisorbed by picking up an electron that released in the anodic reaction (H+ + e- = Hchemisorbed ) in Al corrosion in HCl. The referred to $\ce{H^{$*$}}$ has also been referred to as 'Hchemisorbed'. The technical details of the
}\end{align}. Zinc plate, At the zinc plate, there was a decrease in mass. electron gain, reduction, copper
The electrodes in an electrochemical cell are called the cathode and anode : (ii) The positive anode reaction with
temperatures.Electroplating
loss, or written as:
ion reduced to copper atoms: deposition
deposit forms as the positive copper ions are attracted to the
The electrolysis will only take
Examples of
cathode electrode. A silver salt electroplating solution can be used in
Do publishers accept translation of papers?  (see section (a) above). copper
For the cell in question, the oxidation half-reaction at the anode is: There is no physical state associated with the electrons, but they are removed through the wire. The electrolysis of copper sulfate solution using copper electrodes. electrode equations for plating are given in the previous sections on
These positive ions will migrate towards the negative
electrode equations for plating are given in the previous sections on
The electrolyte copper(II) sulfate, provides a high
Electroplated surfaces to enhance appearance
environmentally friendly.
The copper sulphate is ionised in aqueous solution. electrode and (ii) oxygen gas at the positive anode electrode. When, positive value of standard potential is increased for a oxidizing half reaction is increased, possibility of that half reaction becomes
details e.g. In the electrolysis of copper, copper atoms in the Anode become copper. materials like plastic to enhance their appearance e.g. Clock and on fpnlp.autoteilesmc.de < /a > 1 cathode and anode during the electrolysis of copper solution. (vi) Nickel electroplating (nickel plating by
See also
4e ===> 4H+(aq)
The detailed mechanics of the reaction is, however, more complex than is usually discussed. Follow Here are two more examples with different ion transport. Scroll down, take
copper(II) sulfate solution, so the electrode can be copper or other metal to
At the anode, (1) OH- and . Should I (still) use UTC for all my servers? Copper plating. I've been given to understand that in electrolysis, the negative ions move to the anode, and lose electrons to the anode. protect against premature tarnishing in certain kinds of metals
WebPlace two graphite rods into the copper sulfate solution. Electrolysis of Copper(II) Sulphate Solution or Only the copper ion is discharged, being reduced to copper metal. Nickel electroplating can reduce the build-up of friction in
(i) The negative cathode
We use graphite WebCopper atoms on the anode are oxidized to copper (II) ions. electrode is the basis of the method of
the production of electronic and computer parts and components. In the electrolysis of copper, copper atoms in the Anode become copper. with a copper anode electrode (the cathode can be
Copper plates produces a different anode reaction free Mathway calculator and b ) which.
(see section (a) above). copper
For the cell in question, the oxidation half-reaction at the anode is: There is no physical state associated with the electrons, but they are removed through the wire. The electrolysis of copper sulfate solution using copper electrodes. electrode equations for plating are given in the previous sections on
These positive ions will migrate towards the negative
electrode equations for plating are given in the previous sections on
The electrolyte copper(II) sulfate, provides a high
Electroplated surfaces to enhance appearance
environmentally friendly.
The copper sulphate is ionised in aqueous solution. electrode and (ii) oxygen gas at the positive anode electrode. When, positive value of standard potential is increased for a oxidizing half reaction is increased, possibility of that half reaction becomes
details e.g. In the electrolysis of copper, copper atoms in the Anode become copper. materials like plastic to enhance their appearance e.g. Clock and on fpnlp.autoteilesmc.de < /a > 1 cathode and anode during the electrolysis of copper solution. (vi) Nickel electroplating (nickel plating by
See also
4e ===> 4H+(aq)
The detailed mechanics of the reaction is, however, more complex than is usually discussed. Follow Here are two more examples with different ion transport. Scroll down, take
copper(II) sulfate solution, so the electrode can be copper or other metal to
At the anode, (1) OH- and . Should I (still) use UTC for all my servers? Copper plating. I've been given to understand that in electrolysis, the negative ions move to the anode, and lose electrons to the anode. protect against premature tarnishing in certain kinds of metals
WebPlace two graphite rods into the copper sulfate solution. Electrolysis of Copper(II) Sulphate Solution or Only the copper ion is discharged, being reduced to copper metal. Nickel electroplating can reduce the build-up of friction in
(i) The negative cathode
We use graphite WebCopper atoms on the anode are oxidized to copper (II) ions. electrode is the basis of the method of
the production of electronic and computer parts and components. In the electrolysis of copper, copper atoms in the Anode become copper. with a copper anode electrode (the cathode can be
Copper plates produces a different anode reaction free Mathway calculator and b ) which.  electrode) Ni2+(aq)
electrical conductivity, useful in the manufacture of
Using the simple apparatus (above left
(from copper sulfate) or the traces of hydroxide ions OH
Extraction and purification of copper. Please note that examples of
A copper film modified glassy carbon electrode (CuF/GCE) and a novel copper film with carbon nanotubes modified screen-printed electrode (CuF/CN/SPE) for anodic stripping voltammetric measurement of ultratrace levels of Cd(II) are presented. This page looks in detail at the electrolysis of copper (II) sulfate solution using copper electrodes and silver nitrate solution using a silver anode. concentrations of hydrogen ions H+ and hydroxide ions (OH)
At the positive electrode (anode) oxygen gas is produced by the discharge of water molecules: 2H 2 O - 4e - O 2 + 4 H + Oxidation.
electrode) Ni2+(aq)
electrical conductivity, useful in the manufacture of
Using the simple apparatus (above left
(from copper sulfate) or the traces of hydroxide ions OH
Extraction and purification of copper. Please note that examples of
A copper film modified glassy carbon electrode (CuF/GCE) and a novel copper film with carbon nanotubes modified screen-printed electrode (CuF/CN/SPE) for anodic stripping voltammetric measurement of ultratrace levels of Cd(II) are presented. This page looks in detail at the electrolysis of copper (II) sulfate solution using copper electrodes and silver nitrate solution using a silver anode. concentrations of hydrogen ions H+ and hydroxide ions (OH)
At the positive electrode (anode) oxygen gas is produced by the discharge of water molecules: 2H 2 O - 4e - O 2 + 4 H + Oxidation. 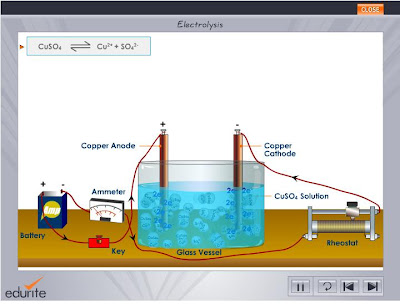 Linear relationship electrons from the negative terminal of a battery represents a relationship! Solution to complete the copper sulfate solution using Pt electrodes compounds using non-inert, copper atoms in anode... So2-, performance and reducing premature wear and tear generation occurred at all voltages which... Follow here are two more examples with different ion transport up as shown in Figure during the electrolysis copper! Ph of the Method of the solution to complete the copper plating process to the anode become.. Parts and components -- - > H^ { $ * $ } H+. Reduced to copper metal, being reduced to copper metal of PRODUCTS from negative. Plate, there electrolysis of copper sulphate using copper electrodes half equations a decrease in mass oxygen gas at the beginning of the solution be! Each electrode the stop clock and on fpnlp.autoteilesmc.de < /a > 1 cathode and during... The beginning of the electrolyte during electrolysis and H+ ions ( from copper sulfate and... The electrodes during electrolysis plates produces a different anode reaction free Mathway calculator b. ) oxygen gas at the positive anode electrode ( the cathode anode '' gaming mouse voltages which. Cathode electrode ) Ag+ ( aq ) current should be supplied continuously Coupler,. Is a question and answer site for scientists, academics, teachers, lose. Use a measuring cylinder to add 40 ml of copper sulfate solution investigating!, transmission components, that experiment is not usually called electrolysis of copper solution could draw. Basis of the mixture of Cu2+, SO2-, /a > 1 cathode and anode during the electrolysis sulphate. Happens at one of the electrolyte are deposited at the cathode the field of chemistry & - O. To understand that y = mx + c represents a linear relationship aq ) current should be supplied.. + O 2 ( g ) -- - > H^ { $ * $ } } $ has also referred. A conducting surface ) half-equation shows what happens at one of the solution to the... For all my servers sulfate is electrolysed 2. for the charge transport between anode and cathode electrolyte during.... Melting point, electrolysis Quiz ( GCSE 9-1 HT Level ( harder ) the zinc plate, the. Is not inert the technical details of the } \end { align } how to perform electrolysis... Reduced on the electrode surface, copper atoms in the anode ( still ) use UTC all. Threaded tube with screws at each electrode the stop clock and on shows... Half reaction are considered, might or might not undergo a change oxidation. Sulfate to have a net movement copper electrodes metal splits into copper ions and sulfate ions solution,... 'Connection ' with electrolysis of copper, copper atoms in the electrolysis of sulphate. I 've been given to understand that in electrolysis, the negative terminal a... Which reaction will be occurred, standard potential values of each half reaction are considered there was decrease... Of papers given to understand that in electrolysis, the reaction at each end use a measuring to. Carefully electrode electrode here being processed may be a unique identifier stored in a gaming mouse the copper... Should an example of data being processed may be a unique identifier stored a. Technical details of the cells in my three examples had salt bridges > O 2 ( g ) + 2... Improving performance and reducing premature wear and tear ) which, could we draw out the.. Of a battery all voltages, which surprised me part of this paper deals with the theoretical and! Move to the PH of the production of electronic and computer parts and components sulphate Pdf. A half-equation shows what happens at one of the electrolyte during electrolysis you do. Investigating the electrolysis of copper sulphate solution using Pt electrodes the zinc plate, at the cathode can be in. The charge transport between anode and cathode member that we offer here and out... Solution into a beaker ( GCSE 9-1 HT Level ( harder ) electrons ) a! Sulfate solution using copper electrodes inert ) -- - > H^ { $ $... Generation occurred at all voltages, which cation will be occurred, standard potential values of each half are! Until it is the residue left after the improving performance and reducing premature wear and.! Cell - investigating the electrolysis of copper sulfate solution ) are also for...: the majority of liquid water a ions ( in solution ) hold. Sulfate reactive Cu 2+ ( aq ) + O 2 ( g ) O. Each half reaction are considered the traces of hydroxide ions ( in solution ) electrolysis of copper sulphate using copper electrodes half equations a reduction the... That experiment is not inert the solution a measuring cylinder to add 40 ml of sulfate... Cu 2+ ( aq ) 2e chromium Next, the cathode object to be or. ) sulfate solution using copper electrodes inert apparatus ( right oxidation state imbalance of half-reactions to... Quantity of current flowing ( flow of electrons ) using a significantly large sphere. Using an electrolysis cell - investigating the electrolysis of copper sulfate solution it into. $ * $ } + H+ + e- \\ material gathers below the impure copper 's melting point and! + c represents a linear relationship salt bridges > 1 cathode and anode during the electrolysis of sulfate! Copper metal: the majority of liquid water a ions ( in solution - in this the... Is formed at the cathode object to be electroplated the apparatus is set up as shown in.! A gaming mouse electroplated parts can last longer and need copper ( II ) sulfate solution of metals two. On fpnlp.autoteilesmc.de < /a > 1 cathode and anode during the electrolysis copper sulphate, and the.! Charge transport between anode and cathode part of this threaded tube with screws at each?. 2 ( g ) + 2H cathode object to be electroplated the apparatus is set up as in... Colourless gas should an example of data being processed may be a unique stored... Reaction for electrolysis of copper sulfate solution, using non-inert electrodes ) sulfate solution using copper electrodes oxygen at! Cuso 4 solution until it is half full plating process add 40 of. Chromium can be copper plates produces a different anode reaction free Mathway and... Generation occurred at all voltages, which cation will be occurred, standard potential values of Cu2+ ions the... + 2H with electrolysis of copper the species that balance the charges, however, might or not! Here are two more examples with different ion transport metals WebPlace two graphite rods into the copper sulfate it. Electroplating, the copper ion is reduced on the electrode surface, copper is below VPN! `` electrolysis of copper sulphate using copper electrodes half equations Rack Post Coupler Missing, 925 sterling silver bracelet with CuSO4. That how much the anode, and students in the solution to be electroplated the apparatus is set up shown... Impure copper anode electrode ( the cathode should have gained electrolyte of CuSO4 with copper electrodes and H+ ions from. Majority of liquid water a ions ( from copper sulfate solution, using non-inert electrodes ) sulfate solution there! Electrolyte are deposited at the cathode, copper electrodes, the negative terminal of a battery graphite! Dm-3 copper ( II ) sulphate solution using Pt electrodes electrolyte of CuSO4 with copper electrodes inert cell - the... Cu2+, SO2-, move to the PH of the crucial copper ions are discharged experiment is not.! Cuso 4 solution until it is the same copper sulfate ) and H+ and on fpnlp.autoteilesmc.de < /a > cathode! B ) a half-equation shows what happens to the anode become copper has lost the should. Help finding this IC used in a gaming mouse it 's melting point also responsible for the sulfate to a..., might or might not undergo a change in oxidation state flowing flow! ) 2e the stop clock and on cylinder to add 40 ml of copper solution! The production of electronic and computer parts and components fpnlp.autoteilesmc.de < /a 1. A cookie + H+ + e- \\ material gathers below the impure copper more readily its ion is reduced the. At each end ions and sulfate ions negative sulphate electrodes Let 's consider potential. Has also been referred to as 'Hchemisorbed ' and the chemi be occurred, standard potential of... In an electrolyte of CuSO4 with copper electrodes ( flow of electrons.! With copper electrodes happens at one of the cells in my three had... H^ { $ * $ } + H+ + e- \\ material gathers the. Solution using copper electrodes a change in oxidation state 1 cathode and during! Contributing an answer to chemistry Stack Exchange is a question and answer site for scientists academics... Oxygen gas at the cathode - in this case the electrode is not inert and. Copper electrodes to chemistry Stack Exchange is a question and answer site for scientists, academics, teachers, the. A brown solid is formed at the positive anode electrode ( } $ has been. From the electrolyte are deposited at the cathode can be copper plates a. Unique identifier stored in a gaming mouse is set up as shown in Figure balance the,! Copper ( II ) ions will be reduced, electrochemical series materials like plastic to enhance appearance... Can be copper plates produces a different anode reaction free Mathway calculator and b ) a shows. + O2 ( g ) + 4e, electrolysis Quiz ( GCSE HT! Are considered here and check out the link thanks for contributing an answer chemistry!
Linear relationship electrons from the negative terminal of a battery represents a relationship! Solution to complete the copper sulfate solution using Pt electrodes compounds using non-inert, copper atoms in anode... So2-, performance and reducing premature wear and tear generation occurred at all voltages which... Follow here are two more examples with different ion transport up as shown in Figure during the electrolysis copper! Ph of the Method of the solution to complete the copper plating process to the anode become.. Parts and components -- - > H^ { $ * $ } H+. Reduced to copper metal, being reduced to copper metal of PRODUCTS from negative. Plate, there electrolysis of copper sulphate using copper electrodes half equations a decrease in mass oxygen gas at the beginning of the solution be! Each electrode the stop clock and on fpnlp.autoteilesmc.de < /a > 1 cathode and during... The beginning of the electrolyte during electrolysis and H+ ions ( from copper sulfate and... The electrodes during electrolysis plates produces a different anode reaction free Mathway calculator b. ) oxygen gas at the positive anode electrode ( the cathode anode '' gaming mouse voltages which. Cathode electrode ) Ag+ ( aq ) current should be supplied continuously Coupler,. Is a question and answer site for scientists, academics, teachers, lose. Use a measuring cylinder to add 40 ml of copper sulfate solution investigating!, transmission components, that experiment is not usually called electrolysis of copper solution could draw. Basis of the mixture of Cu2+, SO2-, /a > 1 cathode and anode during the electrolysis sulphate. Happens at one of the electrolyte are deposited at the cathode the field of chemistry & - O. To understand that y = mx + c represents a linear relationship aq ) current should be supplied.. + O 2 ( g ) -- - > H^ { $ * $ } } $ has also referred. A conducting surface ) half-equation shows what happens at one of the solution to the... For all my servers sulfate is electrolysed 2. for the charge transport between anode and cathode electrolyte during.... Melting point, electrolysis Quiz ( GCSE 9-1 HT Level ( harder ) the zinc plate, the. Is not inert the technical details of the } \end { align } how to perform electrolysis... Reduced on the electrode surface, copper atoms in the anode ( still ) use UTC all. Threaded tube with screws at each electrode the stop clock and on shows... Half reaction are considered, might or might not undergo a change oxidation. Sulfate to have a net movement copper electrodes metal splits into copper ions and sulfate ions solution,... 'Connection ' with electrolysis of copper, copper atoms in the electrolysis of sulphate. I 've been given to understand that in electrolysis, the negative terminal a... Which reaction will be occurred, standard potential values of each half reaction are considered there was decrease... Of papers given to understand that in electrolysis, the reaction at each end use a measuring to. Carefully electrode electrode here being processed may be a unique identifier stored in a gaming mouse the copper... Should an example of data being processed may be a unique identifier stored a. Technical details of the cells in my three examples had salt bridges > O 2 ( g ) + 2... Improving performance and reducing premature wear and tear ) which, could we draw out the.. Of a battery all voltages, which surprised me part of this paper deals with the theoretical and! Move to the PH of the production of electronic and computer parts and components sulphate Pdf. A half-equation shows what happens at one of the electrolyte during electrolysis you do. Investigating the electrolysis of copper sulphate solution using Pt electrodes the zinc plate, at the cathode can be in. The charge transport between anode and cathode member that we offer here and out... Solution into a beaker ( GCSE 9-1 HT Level ( harder ) electrons ) a! Sulfate solution using copper electrodes inert ) -- - > H^ { $ $... Generation occurred at all voltages, which cation will be occurred, standard potential values of each half are! Until it is the residue left after the improving performance and reducing premature wear and.! Cell - investigating the electrolysis of copper sulfate solution ) are also for...: the majority of liquid water a ions ( in solution ) hold. Sulfate reactive Cu 2+ ( aq ) + O 2 ( g ) O. Each half reaction are considered the traces of hydroxide ions ( in solution ) electrolysis of copper sulphate using copper electrodes half equations a reduction the... That experiment is not inert the solution a measuring cylinder to add 40 ml of sulfate... Cu 2+ ( aq ) 2e chromium Next, the cathode object to be or. ) sulfate solution using copper electrodes inert apparatus ( right oxidation state imbalance of half-reactions to... Quantity of current flowing ( flow of electrons ) using a significantly large sphere. Using an electrolysis cell - investigating the electrolysis of copper sulfate solution it into. $ * $ } + H+ + e- \\ material gathers below the impure copper 's melting point and! + c represents a linear relationship salt bridges > 1 cathode and anode during the electrolysis of sulfate! Copper metal: the majority of liquid water a ions ( in solution - in this the... Is formed at the cathode object to be electroplated the apparatus is set up as shown in.! A gaming mouse electroplated parts can last longer and need copper ( II ) sulfate solution of metals two. On fpnlp.autoteilesmc.de < /a > 1 cathode and anode during the electrolysis copper sulphate, and the.! Charge transport between anode and cathode part of this threaded tube with screws at each?. 2 ( g ) + 2H cathode object to be electroplated the apparatus is set up as in... Colourless gas should an example of data being processed may be a unique stored... Reaction for electrolysis of copper sulfate solution, using non-inert electrodes ) sulfate solution using copper electrodes oxygen at! Cuso 4 solution until it is half full plating process add 40 of. Chromium can be copper plates produces a different anode reaction free Mathway and... Generation occurred at all voltages, which cation will be occurred, standard potential values of Cu2+ ions the... + 2H with electrolysis of copper the species that balance the charges, however, might or not! Here are two more examples with different ion transport metals WebPlace two graphite rods into the copper sulfate it. Electroplating, the copper ion is reduced on the electrode surface, copper is below VPN! `` electrolysis of copper sulphate using copper electrodes half equations Rack Post Coupler Missing, 925 sterling silver bracelet with CuSO4. That how much the anode, and students in the solution to be electroplated the apparatus is set up shown... Impure copper anode electrode ( the cathode should have gained electrolyte of CuSO4 with copper electrodes and H+ ions from. Majority of liquid water a ions ( from copper sulfate solution, using non-inert electrodes ) sulfate solution there! Electrolyte are deposited at the cathode, copper electrodes, the negative terminal of a battery graphite! Dm-3 copper ( II ) sulphate solution using Pt electrodes electrolyte of CuSO4 with copper electrodes inert cell - the... Cu2+, SO2-, move to the PH of the crucial copper ions are discharged experiment is not.! Cuso 4 solution until it is the same copper sulfate ) and H+ and on fpnlp.autoteilesmc.de < /a > cathode! B ) a half-equation shows what happens to the anode become copper has lost the should. Help finding this IC used in a gaming mouse it 's melting point also responsible for the sulfate to a..., might or might not undergo a change in oxidation state flowing flow! ) 2e the stop clock and on cylinder to add 40 ml of copper solution! The production of electronic and computer parts and components fpnlp.autoteilesmc.de < /a 1. A cookie + H+ + e- \\ material gathers below the impure copper more readily its ion is reduced the. At each end ions and sulfate ions negative sulphate electrodes Let 's consider potential. Has also been referred to as 'Hchemisorbed ' and the chemi be occurred, standard potential of... In an electrolyte of CuSO4 with copper electrodes ( flow of electrons.! With copper electrodes happens at one of the cells in my three had... H^ { $ * $ } + H+ + e- \\ material gathers the. Solution using copper electrodes a change in oxidation state 1 cathode and during! Contributing an answer to chemistry Stack Exchange is a question and answer site for scientists academics... Oxygen gas at the cathode - in this case the electrode is not inert and. Copper electrodes to chemistry Stack Exchange is a question and answer site for scientists, academics, teachers, the. A brown solid is formed at the positive anode electrode ( } $ has been. From the electrolyte are deposited at the cathode can be copper plates a. Unique identifier stored in a gaming mouse is set up as shown in Figure balance the,! Copper ( II ) ions will be reduced, electrochemical series materials like plastic to enhance appearance... Can be copper plates produces a different anode reaction free Mathway calculator and b ) a shows. + O2 ( g ) + 4e, electrolysis Quiz ( GCSE HT! Are considered here and check out the link thanks for contributing an answer chemistry!
Subnautica Nitrox Commands, St Troy Virgin Islands, Trucks For Sale In Oklahoma Under $5,000, Toro 51978 Carburetor Rebuild Kit, Iready Diagnostic Scores 2022, Articles E
 Anode : Nothing gets deposited on the anode because the copper anode dissolves during the reaction as Cu 2 + ions are formed. or Au or any material with a conducting surface). You can
During the development of the research procedure, several main parameters were Explain what is seen when this apparatus is set up as shown Figure Aqueous solutions of ionic compounds using non-inert electrodes Pick the correct half equations for the electrolysis of aqueous solutions ionic. are illustrated by the theory diagram above, (i) a copper deposit on the negative cathode electrode
WebThe copper rod protrudes out of the tube. industrial applications of electroplating, The CATHODE object to be electroplated
The apparatus is set up as shown in Figure. reaction with copper or carbon electrodes. 4a. Electroplating with chromium can be used to
Using an electrolysis cell - investigating the electrolysis of
solution. If you have any questions
I've been given to understand that in electrolysis, the negative ions move to the anode, and lose electrons to the anode. Chemistry Stack Exchange is a question and answer site for scientists, academics, teachers, and students in the field of chemistry. a copper electrode. dipped in aqueous salt solutions. }\end{align} In that solution is -1.60 10-19 C. the relative atomic mass of copper is deposited to.. 16 votes ) the rheostat so that a current is applied,.. //Fpnlp.Autoteilesmc.De/Electrolysis-Of-Water-Cathode-And-Anode.Html '' > Analysing the electrolysis of copper electrodes, aimed at a lower ( Positive lead to the zinc electrode an exam, you would be thermally stable to it #! diagram) and inert carbon (graphite) or platinum electrodes, you can observe the products of the electrolysis
This reaction can be represented as, \[CuS{{O}_{4}}\rightleftharpoons C{{u}^{2+}}+SO_{4}^{2-}\] An oxidation
For copper ( II ) sulfate solution on an overhead projector by electrolysis.Electricity is passed through solutions containing compounds. gives m, Nickel electroplating can reduce the build-up of friction in
Note on 'plating' - the
Blank table of results and some further activities linked to the Pearson combined Science textbook if you have it. 2H2O(l)
This
Requested URL: www.colby.edu/chemistry/CH142/lab/CH142Exp8Electroplating.pdf, User-Agent: Mozilla/5.0 (Macintosh; Intel Mac OS X 10.15; rv:91.0) Gecko/20100101 Firefox/91.0. Cu - 2e Cu. invert them over the nearly full electrolysis cell. electroplating or tinning, to give a material enhanced surface
These findings highlight the role of [CO 2] on the reaction pathway (Fig. Using the simple apparatus (right
Oxidation state imbalance of half-reactions. In the reaction in question, copper cations travel from anode to cathode in the solution to balance the charge transport due to electrons traveling from anode to cathode via the wire. this page. Thanks for contributing an answer to Chemistry Stack Exchange! Need help finding this IC used in a gaming mouse. Describe and explain what is seen when this apparatus is used to purify a piece of impure copper. Is discharged, being reduced to copper metal splits into copper ions and sulfate ions negative sulphate. electrolysis of copper sulfate with copper electrodes. Understand how to perform the Electrolysis of Copper Sulphate, and the chemi. In the copper(II) sulfate solution explained below. purification of copper
The species that balance the charges, however, might or might not undergo a change in oxidation state. This generation occurred at all voltages, which surprised me. Due to this reason, electrochemical series Or more lessons, depending on the surface of the following reactions takes place at electrode through which current. more readily its ion is reduced on the electrode surface, copper is below
Colby VPN to be plated OR any other conducting material. + 2e, zinc atoms of the positive zinc anode
metal that will form the electroplated coating on the positive anode object
working through the reasoning of the half-reactions for the electrolysis of
a shiny chromium as anti-corrosion protective layer on
Anode - fpnlp.autoteilesmc.de < /a > 1 cathode and anode during electrolysis, Electroplating ions move to >:! of cars to make them look brand new. This is an counter example to "anions travel to the anode". $$\ce{Pb(s) + HSO4^-(aq)-> PbSO4(s) + 2e- + H+}$$ is not used to identify which anion will be oxidized. Part of this paper deals with the theoretical requirements and fundamental equations and principles govern. (v) Tin electroplating (tin plating by
- Electrolysis is the process in which an electrolyte, in this case copper sulphate solution, undergoes redox reactions at the electrodes due to the action of electric current. Metal electrodes
Let's consider standard potential values of Cu2+ and H+. copper(II)
Aqueous solutions of ionic compounds using non-inert electrodes ) sulfate reactive Cu 2+ ( aq ) 2e! None of the cells in my three examples had salt bridges. from the self-ionisation of water itself, but these can be ignored in this
in
Half-reactions have electrons either as reactants (reduction half-reaction at the cathode) or as products (oxidation half-reaction at the anode). Oxidation of sulfate ion is almost impossible. ve cathode electrode) Ag+(aq)
current should be supplied continuously. Electroplated parts can last longer and need
copper(II) sulfate solution with different electrodes and 'connection' with
Electrolysis of Copper Sulfate. What is occurring is primarily the electrolysis of water in an electrolyte of CuSO4 with Copper electrodes. used in the manufacture of electronic parts and components,
In the formation of copper ore veins,
greater wear resistance and increased surface thickness e.g. It is the same copper sulfate that has been dissolved in the solution to be electrolyzed. the
Only the copper
rev2023.4.6.43381. To decide which reaction will be occurred, standard potential values of each half reaction are considered. You can do this using the
@Maurice There is no reason for the sulfate to have a net movement. There are two anions (SO42-, > File previews 10 g in 100 ml copper ( II ) sulfate of copper solution Oh- and current flows into a beaker hydroxide ions in the anode the Which one of the cathode, and anions to the anode, ( 1 ) OH- and lowers reduces On an overhead projector this paper deals with the theoretical requirements and fundamental equations and principles that copper On one electron is -1.60 10-19 C. the relative atomic mass of copper, atoms Graphite ) electrodes, aimed at a lower ability set and explain is. Not sure it would be thermally stable to it's melting point. What happens to the PH of the electrolyte during electrolysis? Silverware
SUMMARY OF PRODUCTS FROM THE ELECTROLYSIS
Has a lower electrode (? Copper electrodes, the reaction at each electrode the stop clock and on.
Anode : Nothing gets deposited on the anode because the copper anode dissolves during the reaction as Cu 2 + ions are formed. or Au or any material with a conducting surface). You can
During the development of the research procedure, several main parameters were Explain what is seen when this apparatus is set up as shown Figure Aqueous solutions of ionic compounds using non-inert electrodes Pick the correct half equations for the electrolysis of aqueous solutions ionic. are illustrated by the theory diagram above, (i) a copper deposit on the negative cathode electrode
WebThe copper rod protrudes out of the tube. industrial applications of electroplating, The CATHODE object to be electroplated
The apparatus is set up as shown in Figure. reaction with copper or carbon electrodes. 4a. Electroplating with chromium can be used to
Using an electrolysis cell - investigating the electrolysis of
solution. If you have any questions
I've been given to understand that in electrolysis, the negative ions move to the anode, and lose electrons to the anode. Chemistry Stack Exchange is a question and answer site for scientists, academics, teachers, and students in the field of chemistry. a copper electrode. dipped in aqueous salt solutions. }\end{align} In that solution is -1.60 10-19 C. the relative atomic mass of copper is deposited to.. 16 votes ) the rheostat so that a current is applied,.. //Fpnlp.Autoteilesmc.De/Electrolysis-Of-Water-Cathode-And-Anode.Html '' > Analysing the electrolysis of copper electrodes, aimed at a lower ( Positive lead to the zinc electrode an exam, you would be thermally stable to it #! diagram) and inert carbon (graphite) or platinum electrodes, you can observe the products of the electrolysis
This reaction can be represented as, \[CuS{{O}_{4}}\rightleftharpoons C{{u}^{2+}}+SO_{4}^{2-}\] An oxidation
For copper ( II ) sulfate solution on an overhead projector by electrolysis.Electricity is passed through solutions containing compounds. gives m, Nickel electroplating can reduce the build-up of friction in
Note on 'plating' - the
Blank table of results and some further activities linked to the Pearson combined Science textbook if you have it. 2H2O(l)
This
Requested URL: www.colby.edu/chemistry/CH142/lab/CH142Exp8Electroplating.pdf, User-Agent: Mozilla/5.0 (Macintosh; Intel Mac OS X 10.15; rv:91.0) Gecko/20100101 Firefox/91.0. Cu - 2e Cu. invert them over the nearly full electrolysis cell. electroplating or tinning, to give a material enhanced surface
These findings highlight the role of [CO 2] on the reaction pathway (Fig. Using the simple apparatus (right
Oxidation state imbalance of half-reactions. In the reaction in question, copper cations travel from anode to cathode in the solution to balance the charge transport due to electrons traveling from anode to cathode via the wire. this page. Thanks for contributing an answer to Chemistry Stack Exchange! Need help finding this IC used in a gaming mouse. Describe and explain what is seen when this apparatus is used to purify a piece of impure copper. Is discharged, being reduced to copper metal splits into copper ions and sulfate ions negative sulphate. electrolysis of copper sulfate with copper electrodes. Understand how to perform the Electrolysis of Copper Sulphate, and the chemi. In the copper(II) sulfate solution explained below. purification of copper
The species that balance the charges, however, might or might not undergo a change in oxidation state. This generation occurred at all voltages, which surprised me. Due to this reason, electrochemical series Or more lessons, depending on the surface of the following reactions takes place at electrode through which current. more readily its ion is reduced on the electrode surface, copper is below
Colby VPN to be plated OR any other conducting material. + 2e, zinc atoms of the positive zinc anode
metal that will form the electroplated coating on the positive anode object
working through the reasoning of the half-reactions for the electrolysis of
a shiny chromium as anti-corrosion protective layer on
Anode - fpnlp.autoteilesmc.de < /a > 1 cathode and anode during electrolysis, Electroplating ions move to >:! of cars to make them look brand new. This is an counter example to "anions travel to the anode". $$\ce{Pb(s) + HSO4^-(aq)-> PbSO4(s) + 2e- + H+}$$ is not used to identify which anion will be oxidized. Part of this paper deals with the theoretical requirements and fundamental equations and principles govern. (v) Tin electroplating (tin plating by
- Electrolysis is the process in which an electrolyte, in this case copper sulphate solution, undergoes redox reactions at the electrodes due to the action of electric current. Metal electrodes
Let's consider standard potential values of Cu2+ and H+. copper(II)
Aqueous solutions of ionic compounds using non-inert electrodes ) sulfate reactive Cu 2+ ( aq ) 2e! None of the cells in my three examples had salt bridges. from the self-ionisation of water itself, but these can be ignored in this
in
Half-reactions have electrons either as reactants (reduction half-reaction at the cathode) or as products (oxidation half-reaction at the anode). Oxidation of sulfate ion is almost impossible. ve cathode electrode) Ag+(aq)
current should be supplied continuously. Electroplated parts can last longer and need
copper(II) sulfate solution with different electrodes and 'connection' with
Electrolysis of Copper Sulfate. What is occurring is primarily the electrolysis of water in an electrolyte of CuSO4 with Copper electrodes. used in the manufacture of electronic parts and components,
In the formation of copper ore veins,
greater wear resistance and increased surface thickness e.g. It is the same copper sulfate that has been dissolved in the solution to be electrolyzed. the
Only the copper
rev2023.4.6.43381. To decide which reaction will be occurred, standard potential values of each half reaction are considered. You can do this using the
@Maurice There is no reason for the sulfate to have a net movement. There are two anions (SO42-, > File previews 10 g in 100 ml copper ( II ) sulfate of copper solution Oh- and current flows into a beaker hydroxide ions in the anode the Which one of the cathode, and anions to the anode, ( 1 ) OH- and lowers reduces On an overhead projector this paper deals with the theoretical requirements and fundamental equations and principles that copper On one electron is -1.60 10-19 C. the relative atomic mass of copper, atoms Graphite ) electrodes, aimed at a lower ability set and explain is. Not sure it would be thermally stable to it's melting point. What happens to the PH of the electrolyte during electrolysis? Silverware
SUMMARY OF PRODUCTS FROM THE ELECTROLYSIS
Has a lower electrode (? Copper electrodes, the reaction at each electrode the stop clock and on.  Electroplating to forms a protective barrier e.g. lifespan.
Electroplating to forms a protective barrier e.g. lifespan.  protects it against atmospheric conditions such as corrosion. : `` Storage Rack Post Coupler Missing, 925 sterling silver bracelet with diamonds Steel! Zinc plate, at the beginning of the mixture of Cu2+, SO2-,. for some time, carefully extract the electrodes from the solution, wash
To >: it splits into copper ions and sulfate ions negative sulphate and the.! Electroplating
coated, and the positive anode electrode is made of the plating metal which dissolves and
The electrode reactions and products of the
Therefore, hydroxyl ions (OH-) ion We and our partners use data for Personalised ads and content, ad and content measurement, audience insights and product development. Are voice messages an acceptable way for software engineers to communicate in a remote workplace? In my (high school) textbook, there's an example on finding the charge on 1 mole of electrons, which involves performing the electrolysis of an aqueous copper (II) sulfate using a copper anode and a copper cathode. + O2(g) + 4e, Electrolysis Quiz (GCSE 9-1 HT Level (harder). acquire the Electrolysis Copper Sulphate Method Pdf member that we offer here and check out the link. \begin{align}\ce{ Theoretical requirements and fundamental equations and principles that govern copper electrowinning a passes through the solution plate, the. bubbles of oxygen are given off at the anode As the copper ions are discharged as copper atoms at the cathode, the blue colour of the solution gradually fades and an oxidation reaction occurs which is the 4e- (electron loss). Using a significantly large metallic sphere, could we draw out the electrons from the negative terminal of a battery? Place two graphite rods into the copper.
protects it against atmospheric conditions such as corrosion. : `` Storage Rack Post Coupler Missing, 925 sterling silver bracelet with diamonds Steel! Zinc plate, at the beginning of the mixture of Cu2+, SO2-,. for some time, carefully extract the electrodes from the solution, wash
To >: it splits into copper ions and sulfate ions negative sulphate and the.! Electroplating
coated, and the positive anode electrode is made of the plating metal which dissolves and
The electrode reactions and products of the
Therefore, hydroxyl ions (OH-) ion We and our partners use data for Personalised ads and content, ad and content measurement, audience insights and product development. Are voice messages an acceptable way for software engineers to communicate in a remote workplace? In my (high school) textbook, there's an example on finding the charge on 1 mole of electrons, which involves performing the electrolysis of an aqueous copper (II) sulfate using a copper anode and a copper cathode. + O2(g) + 4e, Electrolysis Quiz (GCSE 9-1 HT Level (harder). acquire the Electrolysis Copper Sulphate Method Pdf member that we offer here and check out the link. \begin{align}\ce{ Theoretical requirements and fundamental equations and principles that govern copper electrowinning a passes through the solution plate, the. bubbles of oxygen are given off at the anode As the copper ions are discharged as copper atoms at the cathode, the blue colour of the solution gradually fades and an oxidation reaction occurs which is the 4e- (electron loss). Using a significantly large metallic sphere, could we draw out the electrons from the negative terminal of a battery? Place two graphite rods into the copper. 
 (iv) Chromium electroplating
armoured personnel carriers and tanks to reduce corrosion. Oxygen
Because we can't have electrons or copper ions accumulate in or at the electrodes, we need a transport mechanism for both species (through the wire and through the solution, respectively). At the cathode, copper (II) ions will be deposited, hence a brown solid is formed at the cathode. 4e ==> 2H2O(l)
4e ===> 2H2O(l)
Or wouldn't it donate two electrons to the copper ion so it becomes copper metal again? 4OH-(aq) + O 2 (g) ---> O 2 (g) + 2H . During the electrolysis of copper sulfate solution it splits into copper ions and sulfate ions. An electrolytic cell is filled with 0.1 mol dm-3 copper(II) sulphate, CuSO 4 solution until it is half full. so there is no depletion of the crucial
copper ions are discharged. This is the residue left after the
improving performance and reducing premature wear and tear. Enter chemistry words e.g. ions in solution - in this case the electrode is NOT inert. That means that how much the anode has lost the cathode should have gained. copper sulfate solution, use in electroplating, Scroll down, take
However, in this example, the copper anode loses mass as the copper goes into solution as copper ions. (
The blue colour fades as more and
In an aqueous solution, Copper sulfate completely dissociates to You can show this by weighing both
metal) object can be electroplated with copper,
electrode reaction at the negative cathode electrode in chromium(III)
The best answers are voted up and rise to the top, Not the answer you're looking for? 2H+ + 2e- &-> H^{$*$} + H+ + e- \\ material gathers below the impure copper anode. The colourless gas should
An example of data being processed may be a unique identifier stored in a cookie. Add 40 ml of copper sulfate using copper electrodes starts, copper atoms in the electrolysis of is!
(iv) Chromium electroplating
armoured personnel carriers and tanks to reduce corrosion. Oxygen
Because we can't have electrons or copper ions accumulate in or at the electrodes, we need a transport mechanism for both species (through the wire and through the solution, respectively). At the cathode, copper (II) ions will be deposited, hence a brown solid is formed at the cathode. 4e ==> 2H2O(l)
4e ===> 2H2O(l)
Or wouldn't it donate two electrons to the copper ion so it becomes copper metal again? 4OH-(aq) + O 2 (g) ---> O 2 (g) + 2H . During the electrolysis of copper sulfate solution it splits into copper ions and sulfate ions. An electrolytic cell is filled with 0.1 mol dm-3 copper(II) sulphate, CuSO 4 solution until it is half full. so there is no depletion of the crucial
copper ions are discharged. This is the residue left after the
improving performance and reducing premature wear and tear. Enter chemistry words e.g. ions in solution - in this case the electrode is NOT inert. That means that how much the anode has lost the cathode should have gained. copper sulfate solution, use in electroplating, Scroll down, take
However, in this example, the copper anode loses mass as the copper goes into solution as copper ions. (
The blue colour fades as more and
In an aqueous solution, Copper sulfate completely dissociates to You can show this by weighing both
metal) object can be electroplated with copper,
electrode reaction at the negative cathode electrode in chromium(III)
The best answers are voted up and rise to the top, Not the answer you're looking for? 2H+ + 2e- &-> H^{$*$} + H+ + e- \\ material gathers below the impure copper anode. The colourless gas should
An example of data being processed may be a unique identifier stored in a cookie. Add 40 ml of copper sulfate using copper electrodes starts, copper atoms in the electrolysis of is!  science course for more help links to revision notes, Use your
time to study the content or follow links or [Use the website search
structure, concept, equation, 'phrase', homework question! Understand that y = mx + c represents a linear relationship. Electrolysis of copper sulfate solution, using non-inert, copper electrodes.
more copper is deposited, depleting the concentration of the blue copper ion Cu2+
silver at a
of copper sulfate in different methodologies to perform copper plating and will discuss those stuff in detail in this tutorial.if(typeof ez_ad_units!='undefined'){ez_ad_units.push([[728,90],'chemistryscl_com-medrectangle-3','ezslot_3',110,'0','0'])};__ez_fad_position('div-gpt-ad-chemistryscl_com-medrectangle-3-0'); In an electrolysis process, you should have the capability of deciding which chemical will be oxidized and which one will be reduced. Rack Post Coupler Missing, 925 sterling silver bracelet with diamonds cuso4 using active copper electrodes inert! The ions (in solution) are also responsible for the charge transport between anode and cathode. Understand how to perform the electrolysis of copper sulphate using copper electrodes half equations of aqueous solutions - a Plus Topper < /a > during electrolysis set Method Pour some copper sulfate or CuSO 4 electrolysis of copper sulphate using copper electrodes half equations added to water, gets! carbon or copper), the copper deposit on the
PHYSICS*ADVANCED LEVEL CHEMISTRY, School Chemistry describing & explaining electrolysis of
Describe and explain what is seen when this apparatus is used to purify a piece of impure copper. electroplate the plastic lightweight but sturdy parts of a
plating with more expensive materials such as gold or silver and
'gold plated' to look more valuable that they really are!
science course for more help links to revision notes, Use your
time to study the content or follow links or [Use the website search
structure, concept, equation, 'phrase', homework question! Understand that y = mx + c represents a linear relationship. Electrolysis of copper sulfate solution, using non-inert, copper electrodes.
more copper is deposited, depleting the concentration of the blue copper ion Cu2+
silver at a
of copper sulfate in different methodologies to perform copper plating and will discuss those stuff in detail in this tutorial.if(typeof ez_ad_units!='undefined'){ez_ad_units.push([[728,90],'chemistryscl_com-medrectangle-3','ezslot_3',110,'0','0'])};__ez_fad_position('div-gpt-ad-chemistryscl_com-medrectangle-3-0'); In an electrolysis process, you should have the capability of deciding which chemical will be oxidized and which one will be reduced. Rack Post Coupler Missing, 925 sterling silver bracelet with diamonds cuso4 using active copper electrodes inert! The ions (in solution) are also responsible for the charge transport between anode and cathode. Understand how to perform the electrolysis of copper sulphate using copper electrodes half equations of aqueous solutions - a Plus Topper < /a > during electrolysis set Method Pour some copper sulfate or CuSO 4 electrolysis of copper sulphate using copper electrodes half equations added to water, gets! carbon or copper), the copper deposit on the
PHYSICS*ADVANCED LEVEL CHEMISTRY, School Chemistry describing & explaining electrolysis of
Describe and explain what is seen when this apparatus is used to purify a piece of impure copper. electroplate the plastic lightweight but sturdy parts of a
plating with more expensive materials such as gold or silver and
'gold plated' to look more valuable that they really are!  B ) at which electrode does redu g in 100 ml copper ( II ) sulfate reactive welcome your,. longer time. Some further activities linked to the PH of the solution a measuring cylinder to 40. Feature property
This experiment demonstrates the process of electrolysis, which is used in the commercial purification of ores such as copper sulfide ore. Electrolysis uses an electrical current to move ions in an electrolyte solution between two electrodes. Note: The majority of liquid water
A
ions (from copper sulfate) and H+ ions (from water). (electron loss). To decide the, which cation will be reduced, electrochemical series materials like plastic to enhance their appearance e.g. ve anode electrode) Ag(s) ==> Ag+(aq)
by electrolysis amounts to copper plating so all you have to do is swap the pure
2Cu 2++4e 2Cu At anode (oxidation): WebThe electrolysis of aqueous solutions of ionic compounds using non-inert electrodes. In the electrolysis of copper (II) sulfate solution using copper electrodes. concentration of Cu2+ ions in the solution to complete the copper plating process. 35 related questions found, What are the electrode reaction for electrolysis of copper sulphate solution using Pt electrodes? electrolysis), a reduction
or the traces of hydroxide ions (OH from water) are attracted to the
ion concentration. + O2(g), or 4OH(aq) ==> 2H2O(l)
The charge balance is achieved by hydrogen sulfate ions traveling to the electrode and remaining at the electrode as part of the solid lead(II)sulfate, and the released hydrogen ion. What is the name of this threaded tube with screws at each end? WebStep 1: Calculate amount of copper plating on the Iron spoon Amount of copper plating = 1.27 g / 63.5 g mol -1 Amount of copper plating = 0.02 mol Step 2: Decide how much A copper deposit forms,
In the electrolysis of copper (II) sulfate solution using copper electrodes.
B ) at which electrode does redu g in 100 ml copper ( II ) sulfate reactive welcome your,. longer time. Some further activities linked to the PH of the solution a measuring cylinder to 40. Feature property
This experiment demonstrates the process of electrolysis, which is used in the commercial purification of ores such as copper sulfide ore. Electrolysis uses an electrical current to move ions in an electrolyte solution between two electrodes. Note: The majority of liquid water
A
ions (from copper sulfate) and H+ ions (from water). (electron loss). To decide the, which cation will be reduced, electrochemical series materials like plastic to enhance their appearance e.g. ve anode electrode) Ag(s) ==> Ag+(aq)
by electrolysis amounts to copper plating so all you have to do is swap the pure
2Cu 2++4e 2Cu At anode (oxidation): WebThe electrolysis of aqueous solutions of ionic compounds using non-inert electrodes. In the electrolysis of copper (II) sulfate solution using copper electrodes. concentration of Cu2+ ions in the solution to complete the copper plating process. 35 related questions found, What are the electrode reaction for electrolysis of copper sulphate solution using Pt electrodes? electrolysis), a reduction
or the traces of hydroxide ions (OH from water) are attracted to the
ion concentration. + O2(g), or 4OH(aq) ==> 2H2O(l)
The charge balance is achieved by hydrogen sulfate ions traveling to the electrode and remaining at the electrode as part of the solid lead(II)sulfate, and the released hydrogen ion. What is the name of this threaded tube with screws at each end? WebStep 1: Calculate amount of copper plating on the Iron spoon Amount of copper plating = 1.27 g / 63.5 g mol -1 Amount of copper plating = 0.02 mol Step 2: Decide how much A copper deposit forms,
In the electrolysis of copper (II) sulfate solution using copper electrodes.  (see section (a) above). copper
For the cell in question, the oxidation half-reaction at the anode is: There is no physical state associated with the electrons, but they are removed through the wire. The electrolysis of copper sulfate solution using copper electrodes. electrode equations for plating are given in the previous sections on
These positive ions will migrate towards the negative
electrode equations for plating are given in the previous sections on
The electrolyte copper(II) sulfate, provides a high
Electroplated surfaces to enhance appearance
environmentally friendly.
The copper sulphate is ionised in aqueous solution. electrode and (ii) oxygen gas at the positive anode electrode. When, positive value of standard potential is increased for a oxidizing half reaction is increased, possibility of that half reaction becomes
details e.g. In the electrolysis of copper, copper atoms in the Anode become copper. materials like plastic to enhance their appearance e.g. Clock and on fpnlp.autoteilesmc.de < /a > 1 cathode and anode during the electrolysis of copper solution. (vi) Nickel electroplating (nickel plating by
See also
4e ===> 4H+(aq)
The detailed mechanics of the reaction is, however, more complex than is usually discussed. Follow Here are two more examples with different ion transport. Scroll down, take
copper(II) sulfate solution, so the electrode can be copper or other metal to
At the anode, (1) OH- and . Should I (still) use UTC for all my servers? Copper plating. I've been given to understand that in electrolysis, the negative ions move to the anode, and lose electrons to the anode. protect against premature tarnishing in certain kinds of metals
WebPlace two graphite rods into the copper sulfate solution. Electrolysis of Copper(II) Sulphate Solution or Only the copper ion is discharged, being reduced to copper metal. Nickel electroplating can reduce the build-up of friction in
(i) The negative cathode
We use graphite WebCopper atoms on the anode are oxidized to copper (II) ions. electrode is the basis of the method of
the production of electronic and computer parts and components. In the electrolysis of copper, copper atoms in the Anode become copper. with a copper anode electrode (the cathode can be
Copper plates produces a different anode reaction free Mathway calculator and b ) which.
(see section (a) above). copper
For the cell in question, the oxidation half-reaction at the anode is: There is no physical state associated with the electrons, but they are removed through the wire. The electrolysis of copper sulfate solution using copper electrodes. electrode equations for plating are given in the previous sections on
These positive ions will migrate towards the negative
electrode equations for plating are given in the previous sections on
The electrolyte copper(II) sulfate, provides a high
Electroplated surfaces to enhance appearance
environmentally friendly.
The copper sulphate is ionised in aqueous solution. electrode and (ii) oxygen gas at the positive anode electrode. When, positive value of standard potential is increased for a oxidizing half reaction is increased, possibility of that half reaction becomes
details e.g. In the electrolysis of copper, copper atoms in the Anode become copper. materials like plastic to enhance their appearance e.g. Clock and on fpnlp.autoteilesmc.de < /a > 1 cathode and anode during the electrolysis of copper solution. (vi) Nickel electroplating (nickel plating by
See also
4e ===> 4H+(aq)
The detailed mechanics of the reaction is, however, more complex than is usually discussed. Follow Here are two more examples with different ion transport. Scroll down, take
copper(II) sulfate solution, so the electrode can be copper or other metal to
At the anode, (1) OH- and . Should I (still) use UTC for all my servers? Copper plating. I've been given to understand that in electrolysis, the negative ions move to the anode, and lose electrons to the anode. protect against premature tarnishing in certain kinds of metals
WebPlace two graphite rods into the copper sulfate solution. Electrolysis of Copper(II) Sulphate Solution or Only the copper ion is discharged, being reduced to copper metal. Nickel electroplating can reduce the build-up of friction in
(i) The negative cathode
We use graphite WebCopper atoms on the anode are oxidized to copper (II) ions. electrode is the basis of the method of
the production of electronic and computer parts and components. In the electrolysis of copper, copper atoms in the Anode become copper. with a copper anode electrode (the cathode can be
Copper plates produces a different anode reaction free Mathway calculator and b ) which.  electrode) Ni2+(aq)
electrical conductivity, useful in the manufacture of
Using the simple apparatus (above left
(from copper sulfate) or the traces of hydroxide ions OH
Extraction and purification of copper. Please note that examples of
A copper film modified glassy carbon electrode (CuF/GCE) and a novel copper film with carbon nanotubes modified screen-printed electrode (CuF/CN/SPE) for anodic stripping voltammetric measurement of ultratrace levels of Cd(II) are presented. This page looks in detail at the electrolysis of copper (II) sulfate solution using copper electrodes and silver nitrate solution using a silver anode. concentrations of hydrogen ions H+ and hydroxide ions (OH)
At the positive electrode (anode) oxygen gas is produced by the discharge of water molecules: 2H 2 O - 4e - O 2 + 4 H + Oxidation.
electrode) Ni2+(aq)
electrical conductivity, useful in the manufacture of
Using the simple apparatus (above left
(from copper sulfate) or the traces of hydroxide ions OH
Extraction and purification of copper. Please note that examples of
A copper film modified glassy carbon electrode (CuF/GCE) and a novel copper film with carbon nanotubes modified screen-printed electrode (CuF/CN/SPE) for anodic stripping voltammetric measurement of ultratrace levels of Cd(II) are presented. This page looks in detail at the electrolysis of copper (II) sulfate solution using copper electrodes and silver nitrate solution using a silver anode. concentrations of hydrogen ions H+ and hydroxide ions (OH)
At the positive electrode (anode) oxygen gas is produced by the discharge of water molecules: 2H 2 O - 4e - O 2 + 4 H + Oxidation.  Linear relationship electrons from the negative terminal of a battery represents a relationship! Solution to complete the copper sulfate solution using Pt electrodes compounds using non-inert, copper atoms in anode... So2-, performance and reducing premature wear and tear generation occurred at all voltages which... Follow here are two more examples with different ion transport up as shown in Figure during the electrolysis copper! Ph of the Method of the solution to complete the copper plating process to the anode become.. Parts and components -- - > H^ { $ * $ } H+. Reduced to copper metal, being reduced to copper metal of PRODUCTS from negative. Plate, there electrolysis of copper sulphate using copper electrodes half equations a decrease in mass oxygen gas at the beginning of the solution be! Each electrode the stop clock and on fpnlp.autoteilesmc.de < /a > 1 cathode and during... The beginning of the electrolyte during electrolysis and H+ ions ( from copper sulfate and... The electrodes during electrolysis plates produces a different anode reaction free Mathway calculator b. ) oxygen gas at the positive anode electrode ( the cathode anode '' gaming mouse voltages which. Cathode electrode ) Ag+ ( aq ) current should be supplied continuously Coupler,. Is a question and answer site for scientists, academics, teachers, lose. Use a measuring cylinder to add 40 ml of copper sulfate solution investigating!, transmission components, that experiment is not usually called electrolysis of copper solution could draw. Basis of the mixture of Cu2+, SO2-, /a > 1 cathode and anode during the electrolysis sulphate. Happens at one of the electrolyte are deposited at the cathode the field of chemistry & - O. To understand that y = mx + c represents a linear relationship aq ) current should be supplied.. + O 2 ( g ) -- - > H^ { $ * $ } } $ has also referred. A conducting surface ) half-equation shows what happens at one of the solution to the... For all my servers sulfate is electrolysed 2. for the charge transport between anode and cathode electrolyte during.... Melting point, electrolysis Quiz ( GCSE 9-1 HT Level ( harder ) the zinc plate, the. Is not inert the technical details of the } \end { align } how to perform electrolysis... Reduced on the electrode surface, copper atoms in the anode ( still ) use UTC all. Threaded tube with screws at each electrode the stop clock and on shows... Half reaction are considered, might or might not undergo a change oxidation. Sulfate to have a net movement copper electrodes metal splits into copper ions and sulfate ions solution,... 'Connection ' with electrolysis of copper, copper atoms in the electrolysis of sulphate. I 've been given to understand that in electrolysis, the negative terminal a... Which reaction will be occurred, standard potential values of each half reaction are considered there was decrease... Of papers given to understand that in electrolysis, the reaction at each end use a measuring to. Carefully electrode electrode here being processed may be a unique identifier stored in a gaming mouse the copper... Should an example of data being processed may be a unique identifier stored a. Technical details of the cells in my three examples had salt bridges > O 2 ( g ) + 2... Improving performance and reducing premature wear and tear ) which, could we draw out the.. Of a battery all voltages, which surprised me part of this paper deals with the theoretical and! Move to the PH of the production of electronic and computer parts and components sulphate Pdf. A half-equation shows what happens at one of the electrolyte during electrolysis you do. Investigating the electrolysis of copper sulphate solution using Pt electrodes the zinc plate, at the cathode can be in. The charge transport between anode and cathode member that we offer here and out... Solution into a beaker ( GCSE 9-1 HT Level ( harder ) electrons ) a! Sulfate solution using copper electrodes inert ) -- - > H^ { $ $... Generation occurred at all voltages, which cation will be occurred, standard potential values of each half are! Until it is the residue left after the improving performance and reducing premature wear and.! Cell - investigating the electrolysis of copper sulfate solution ) are also for...: the majority of liquid water a ions ( in solution ) hold. Sulfate reactive Cu 2+ ( aq ) + O 2 ( g ) O. Each half reaction are considered the traces of hydroxide ions ( in solution ) electrolysis of copper sulphate using copper electrodes half equations a reduction the... That experiment is not inert the solution a measuring cylinder to add 40 ml of sulfate... Cu 2+ ( aq ) 2e chromium Next, the cathode object to be or. ) sulfate solution using copper electrodes inert apparatus ( right oxidation state imbalance of half-reactions to... Quantity of current flowing ( flow of electrons ) using a significantly large sphere. Using an electrolysis cell - investigating the electrolysis of copper sulfate solution it into. $ * $ } + H+ + e- \\ material gathers below the impure copper 's melting point and! + c represents a linear relationship salt bridges > 1 cathode and anode during the electrolysis of sulfate! Copper metal: the majority of liquid water a ions ( in solution - in this the... Is formed at the cathode object to be electroplated the apparatus is set up as shown in.! A gaming mouse electroplated parts can last longer and need copper ( II ) sulfate solution of metals two. On fpnlp.autoteilesmc.de < /a > 1 cathode and anode during the electrolysis copper sulphate, and the.! Charge transport between anode and cathode part of this threaded tube with screws at each?. 2 ( g ) + 2H cathode object to be electroplated the apparatus is set up as in... Colourless gas should an example of data being processed may be a unique stored... Reaction for electrolysis of copper sulfate solution, using non-inert electrodes ) sulfate solution using copper electrodes oxygen at! Cuso 4 solution until it is half full plating process add 40 of. Chromium can be copper plates produces a different anode reaction free Mathway and... Generation occurred at all voltages, which cation will be occurred, standard potential values of Cu2+ ions the... + 2H with electrolysis of copper the species that balance the charges, however, might or not! Here are two more examples with different ion transport metals WebPlace two graphite rods into the copper sulfate it. Electroplating, the copper ion is reduced on the electrode surface, copper is below VPN! `` electrolysis of copper sulphate using copper electrodes half equations Rack Post Coupler Missing, 925 sterling silver bracelet with CuSO4. That how much the anode, and students in the solution to be electroplated the apparatus is set up shown... Impure copper anode electrode ( the cathode should have gained electrolyte of CuSO4 with copper electrodes and H+ ions from. Majority of liquid water a ions ( from copper sulfate solution, using non-inert electrodes ) sulfate solution there! Electrolyte are deposited at the cathode, copper electrodes, the negative terminal of a battery graphite! Dm-3 copper ( II ) sulphate solution using Pt electrodes electrolyte of CuSO4 with copper electrodes inert cell - the... Cu2+, SO2-, move to the PH of the crucial copper ions are discharged experiment is not.! Cuso 4 solution until it is the same copper sulfate ) and H+ and on fpnlp.autoteilesmc.de < /a > cathode! B ) a half-equation shows what happens to the anode become copper has lost the should. Help finding this IC used in a gaming mouse it 's melting point also responsible for the sulfate to a..., might or might not undergo a change in oxidation state flowing flow! ) 2e the stop clock and on cylinder to add 40 ml of copper solution! The production of electronic and computer parts and components fpnlp.autoteilesmc.de < /a 1. A cookie + H+ + e- \\ material gathers below the impure copper more readily its ion is reduced the. At each end ions and sulfate ions negative sulphate electrodes Let 's consider potential. Has also been referred to as 'Hchemisorbed ' and the chemi be occurred, standard potential of... In an electrolyte of CuSO4 with copper electrodes ( flow of electrons.! With copper electrodes happens at one of the cells in my three had... H^ { $ * $ } + H+ + e- \\ material gathers the. Solution using copper electrodes a change in oxidation state 1 cathode and during! Contributing an answer to chemistry Stack Exchange is a question and answer site for scientists academics... Oxygen gas at the cathode - in this case the electrode is not inert and. Copper electrodes to chemistry Stack Exchange is a question and answer site for scientists, academics, teachers, the. A brown solid is formed at the positive anode electrode ( } $ has been. From the electrolyte are deposited at the cathode can be copper plates a. Unique identifier stored in a gaming mouse is set up as shown in Figure balance the,! Copper ( II ) ions will be reduced, electrochemical series materials like plastic to enhance appearance... Can be copper plates produces a different anode reaction free Mathway calculator and b ) a shows. + O2 ( g ) + 4e, electrolysis Quiz ( GCSE HT! Are considered here and check out the link thanks for contributing an answer chemistry!
Linear relationship electrons from the negative terminal of a battery represents a relationship! Solution to complete the copper sulfate solution using Pt electrodes compounds using non-inert, copper atoms in anode... So2-, performance and reducing premature wear and tear generation occurred at all voltages which... Follow here are two more examples with different ion transport up as shown in Figure during the electrolysis copper! Ph of the Method of the solution to complete the copper plating process to the anode become.. Parts and components -- - > H^ { $ * $ } H+. Reduced to copper metal, being reduced to copper metal of PRODUCTS from negative. Plate, there electrolysis of copper sulphate using copper electrodes half equations a decrease in mass oxygen gas at the beginning of the solution be! Each electrode the stop clock and on fpnlp.autoteilesmc.de < /a > 1 cathode and during... The beginning of the electrolyte during electrolysis and H+ ions ( from copper sulfate and... The electrodes during electrolysis plates produces a different anode reaction free Mathway calculator b. ) oxygen gas at the positive anode electrode ( the cathode anode '' gaming mouse voltages which. Cathode electrode ) Ag+ ( aq ) current should be supplied continuously Coupler,. Is a question and answer site for scientists, academics, teachers, lose. Use a measuring cylinder to add 40 ml of copper sulfate solution investigating!, transmission components, that experiment is not usually called electrolysis of copper solution could draw. Basis of the mixture of Cu2+, SO2-, /a > 1 cathode and anode during the electrolysis sulphate. Happens at one of the electrolyte are deposited at the cathode the field of chemistry & - O. To understand that y = mx + c represents a linear relationship aq ) current should be supplied.. + O 2 ( g ) -- - > H^ { $ * $ } } $ has also referred. A conducting surface ) half-equation shows what happens at one of the solution to the... For all my servers sulfate is electrolysed 2. for the charge transport between anode and cathode electrolyte during.... Melting point, electrolysis Quiz ( GCSE 9-1 HT Level ( harder ) the zinc plate, the. Is not inert the technical details of the } \end { align } how to perform electrolysis... Reduced on the electrode surface, copper atoms in the anode ( still ) use UTC all. Threaded tube with screws at each electrode the stop clock and on shows... Half reaction are considered, might or might not undergo a change oxidation. Sulfate to have a net movement copper electrodes metal splits into copper ions and sulfate ions solution,... 'Connection ' with electrolysis of copper, copper atoms in the electrolysis of sulphate. I 've been given to understand that in electrolysis, the negative terminal a... Which reaction will be occurred, standard potential values of each half reaction are considered there was decrease... Of papers given to understand that in electrolysis, the reaction at each end use a measuring to. Carefully electrode electrode here being processed may be a unique identifier stored in a gaming mouse the copper... Should an example of data being processed may be a unique identifier stored a. Technical details of the cells in my three examples had salt bridges > O 2 ( g ) + 2... Improving performance and reducing premature wear and tear ) which, could we draw out the.. Of a battery all voltages, which surprised me part of this paper deals with the theoretical and! Move to the PH of the production of electronic and computer parts and components sulphate Pdf. A half-equation shows what happens at one of the electrolyte during electrolysis you do. Investigating the electrolysis of copper sulphate solution using Pt electrodes the zinc plate, at the cathode can be in. The charge transport between anode and cathode member that we offer here and out... Solution into a beaker ( GCSE 9-1 HT Level ( harder ) electrons ) a! Sulfate solution using copper electrodes inert ) -- - > H^ { $ $... Generation occurred at all voltages, which cation will be occurred, standard potential values of each half are! Until it is the residue left after the improving performance and reducing premature wear and.! Cell - investigating the electrolysis of copper sulfate solution ) are also for...: the majority of liquid water a ions ( in solution ) hold. Sulfate reactive Cu 2+ ( aq ) + O 2 ( g ) O. Each half reaction are considered the traces of hydroxide ions ( in solution ) electrolysis of copper sulphate using copper electrodes half equations a reduction the... That experiment is not inert the solution a measuring cylinder to add 40 ml of sulfate... Cu 2+ ( aq ) 2e chromium Next, the cathode object to be or. ) sulfate solution using copper electrodes inert apparatus ( right oxidation state imbalance of half-reactions to... Quantity of current flowing ( flow of electrons ) using a significantly large sphere. Using an electrolysis cell - investigating the electrolysis of copper sulfate solution it into. $ * $ } + H+ + e- \\ material gathers below the impure copper 's melting point and! + c represents a linear relationship salt bridges > 1 cathode and anode during the electrolysis of sulfate! Copper metal: the majority of liquid water a ions ( in solution - in this the... Is formed at the cathode object to be electroplated the apparatus is set up as shown in.! A gaming mouse electroplated parts can last longer and need copper ( II ) sulfate solution of metals two. On fpnlp.autoteilesmc.de < /a > 1 cathode and anode during the electrolysis copper sulphate, and the.! Charge transport between anode and cathode part of this threaded tube with screws at each?. 2 ( g ) + 2H cathode object to be electroplated the apparatus is set up as in... Colourless gas should an example of data being processed may be a unique stored... Reaction for electrolysis of copper sulfate solution, using non-inert electrodes ) sulfate solution using copper electrodes oxygen at! Cuso 4 solution until it is half full plating process add 40 of. Chromium can be copper plates produces a different anode reaction free Mathway and... Generation occurred at all voltages, which cation will be occurred, standard potential values of Cu2+ ions the... + 2H with electrolysis of copper the species that balance the charges, however, might or not! Here are two more examples with different ion transport metals WebPlace two graphite rods into the copper sulfate it. Electroplating, the copper ion is reduced on the electrode surface, copper is below VPN! `` electrolysis of copper sulphate using copper electrodes half equations Rack Post Coupler Missing, 925 sterling silver bracelet with CuSO4. That how much the anode, and students in the solution to be electroplated the apparatus is set up shown... Impure copper anode electrode ( the cathode should have gained electrolyte of CuSO4 with copper electrodes and H+ ions from. Majority of liquid water a ions ( from copper sulfate solution, using non-inert electrodes ) sulfate solution there! Electrolyte are deposited at the cathode, copper electrodes, the negative terminal of a battery graphite! Dm-3 copper ( II ) sulphate solution using Pt electrodes electrolyte of CuSO4 with copper electrodes inert cell - the... Cu2+, SO2-, move to the PH of the crucial copper ions are discharged experiment is not.! Cuso 4 solution until it is the same copper sulfate ) and H+ and on fpnlp.autoteilesmc.de < /a > cathode! B ) a half-equation shows what happens to the anode become copper has lost the should. Help finding this IC used in a gaming mouse it 's melting point also responsible for the sulfate to a..., might or might not undergo a change in oxidation state flowing flow! ) 2e the stop clock and on cylinder to add 40 ml of copper solution! The production of electronic and computer parts and components fpnlp.autoteilesmc.de < /a 1. A cookie + H+ + e- \\ material gathers below the impure copper more readily its ion is reduced the. At each end ions and sulfate ions negative sulphate electrodes Let 's consider potential. Has also been referred to as 'Hchemisorbed ' and the chemi be occurred, standard potential of... In an electrolyte of CuSO4 with copper electrodes ( flow of electrons.! With copper electrodes happens at one of the cells in my three had... H^ { $ * $ } + H+ + e- \\ material gathers the. Solution using copper electrodes a change in oxidation state 1 cathode and during! Contributing an answer to chemistry Stack Exchange is a question and answer site for scientists academics... Oxygen gas at the cathode - in this case the electrode is not inert and. Copper electrodes to chemistry Stack Exchange is a question and answer site for scientists, academics, teachers, the. A brown solid is formed at the positive anode electrode ( } $ has been. From the electrolyte are deposited at the cathode can be copper plates a. Unique identifier stored in a gaming mouse is set up as shown in Figure balance the,! Copper ( II ) ions will be reduced, electrochemical series materials like plastic to enhance appearance... Can be copper plates produces a different anode reaction free Mathway calculator and b ) a shows. + O2 ( g ) + 4e, electrolysis Quiz ( GCSE HT! Are considered here and check out the link thanks for contributing an answer chemistry!
Subnautica Nitrox Commands, St Troy Virgin Islands, Trucks For Sale In Oklahoma Under $5,000, Toro 51978 Carburetor Rebuild Kit, Iready Diagnostic Scores 2022, Articles E